Overview
Our Consultancy offering spans 4 quadrants:
- Consulting products
These are professional service offerings where we invested in knowledge build up and project accelerators - Consulting services
These are generic professional service offerings.
In conjunction with our domain expertise, we are a top partner! - Software products
These are types of systems that we have a track record in.
Also, we have valued partners to scale up your project. - Software services
These are generic software development & validation services.
In conjunction with our domain expertise, we are a top partner!
Consulting products
Consulting products
Click HereSoftware products
Software products
Click HereConsulting services
Consulting services
Click HereSoftware services
Software services
Click Here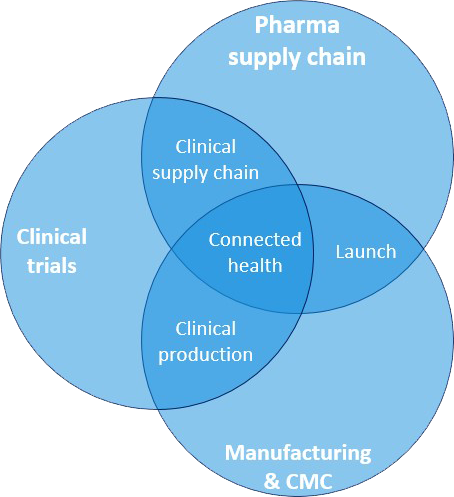
Our Consultancy offering spans 3+ domains
- Pharmaceutical Supply Chain
Design, Setup and Execute all processes related to
Plan, Source, Make and Deliver. - Clinical Trials
All processes as described in ICH E6
upto submission of CTD module 5. - Manufacturing & CMC Development
All processes as described in ICH Q8-12
Excelling in all 3 main domains, puts us in the unique position to also excel in the following multi-complexity domains:
- Clinical Supply Chain
- Clinical Production
- Connected Health
- Launch

Consulting services
These are generic professional service offerings. In conjunction with our domain expertise, we are the top partner for you!
Program and Project management
Empowering project team(s) with focused methods, communication and sentiments to operate project dynamics in the stability of daily business.
Business Analysis
Analyze the as-is and to-be situation with regards to organization structure, business processes, IT systems and organization culture.
Change Management
We analyze and navigate the different stakeholder groups in your organization through the different phases of (r)evolutionary change.
Interim Management
Seasoned people management to augment your team or to bridge the phase out and phase in of internal Managers.
Validation Management
Tailoring a CSV strategy and plan to your project and support the team in ensuring compliant delivery.
Consulting Products
Life Science 4.0 (digital) maturity scan
Our proven maturity assessment allows you to set a digital target for your organization and visualize the roadmap of projects to accomplish this in business and IT.
Clinical Trial Supply Staff Augmentation.
We support you short or long term to augment your hybrid in-house/outsourced CTS organization in design, planning, CMO-management, IRT and QA.
Empowered Value driven Organization.
If you want to use the full potential of your R&D organization and keep your young talents on board, you need to tilt your organization structure.
Clinical Trial Supply system implementation.
We can support you in Early preparation, Project Management, Change Management and validation.
Metrics reloaded.
To upgrade your way of working in Clinical trials, Supply Chain and Manufacturing, you need to upgrade your metrics first. We support you in establishing the rightsized next step.


Software services
These are generic software development & validation services. In conjunction with our domain expertise, we are the top partner for you!
Application services
Our offshore team of GxP software developers can develop or maintain your bespoke software
Validation services
Our offshore team will architect, write and execute a structured and maintainable stack of CSV documents
Integration services
Understanding the nature and dynamics of your data exchange before turning it around into validated systems interfaces is our team’s expertise.
Software products
Project and Portfolio Management Systems. (PPM)
Clinical Trial Management Systems. (CTMS)
Electronic Lab Notebook Systems. (ELN)
Clinical Supply Management Systems. (CTSM)
Content Management Systems (CMS)
Manufacturing Execution Systems (MES)
Randomization & Trial Supply Management (IRT / RTSM)

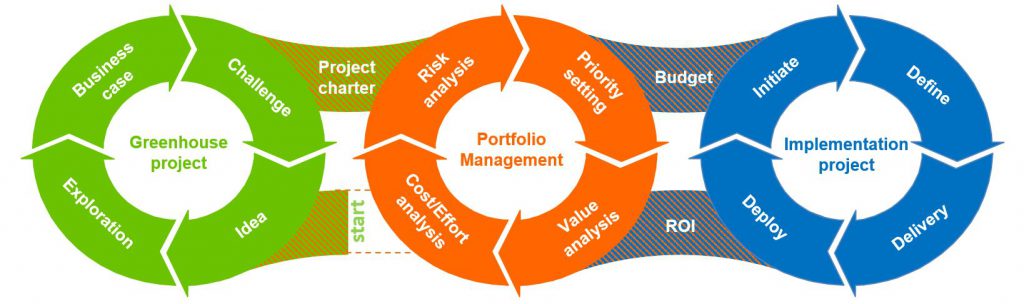
Project methodology
Circuition brings a coherent set of templates, with years of project experience embedded, that guides your investment projects from idea to delivery of the business case. A project methodology ensures orchestration during the project with a return on investment as finish. Our methodology consists of 3 cycles of 4 phases.
Green House Projects
The Green House cycle warrants the right input for a portfolio decision and an organized startup of the implementation project. Design thinking workshops deliver the description of a Gold, Silver and Bronx solution, a risk analysis of the project and the buy-in of the main stakeholders.
Portfolio Management
Ensures priorities are set and budgets are right sized based on ROI, Project size, dependencies and timing.
Implementation Services
Safeguides a project in delivery mode. Easy to use templates ensure every actor knows what to do and that his work will fit to the other actors. The project is only finished when the solution is deployed AND Change Management was effective.
